Abstract
Background: Health status in patients with hemophilia (PWH) are affected by bleeding related complications, for instance, intermittent joint bleeding, hemophilic arthropathy and chronic pain. Aged PWH are also found to have comorbid diseases which impact their health status. Optimal care for PWH requires an integrated and multidisciplinary approaches. The aim of this study is to evaluate the burden of comorbid diseases in PWH.
Methods: We performed a cross-sectional analysis of respondents who participated to the phase 1 (17 countries) and 2b (6 countries) of the PROBE study. All included participants were asked to answer the 29-item PROBE questionnaire. We calculated the prevalence of comorbid diseases reported by the participants including, hepatitis, stroke, hypertension, angina, heart attack, liver cancer, other cancer, diabetes, seizure, arthritis, gingivitis and HIV infection. Aged adjusted odds ratios of the prevalence of comorbid diseases in PWH were calculated as compared to participants without bleeding disorders.
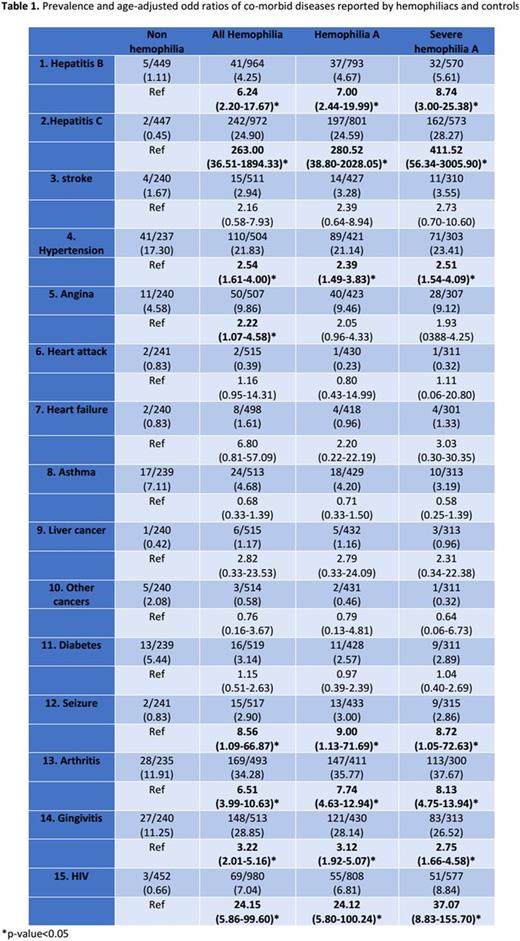
Results: There were 1170 PWHs and 525 participants without bleeding disorders included in the analysis. Mean age of participants was lower in PWHs group (34.18±17.84 vs 44.80±13.77). Among PWHs, 84.27% were hemophilia A and 15.73% were hemophilia B. With regards to severity of hemophilia 13.93% were mild, 17.81% were moderate and 66.27% were severe. Table 1 demonstrates prevalence of comorbid diseases in participants. PWHs were associated with higher prevalence of hepatitis B (OR 6.2, 95%CI 2.2-17.7), hepatitis C (OR 263.0, 95%CI 36.5-1894.3), HIV (OR 24.2 95%CI 5.9-99.6), hypertension (OR 2.5, 95%CI 1.6-4.0), angina (OR 2.2, 95%CI 1.07-4.6), seizure (OR 8.6, 95%CI 1.1-66.9), arthritis (OR 6.5, 95%CI 4.0-10.6) and gingivitis (OR 3.2, 95%CI 2.0-5.2).
Conclusion: When compared to participants without bleeding disorders, PWHs frequently reported hemophilia related diseases (hepatitis B, C and HIV infection and arthritis). Moreover, PWHs were associated with higher prevalence of hypertension and gingivitis across all disease severity. These findings suggested that selective comorbid diseases assessment in PWHs should be incorporated in usual hemophilia care.
Skinner: Baxalta, now part of Shire; Bayer; Bioverativ; CSL; Novo Nordisk, Roche and Sobi with administrative support provided by the US National Hemophilia Foundation: Research Funding; US National Hemophilia Foundation: Other: non-financial support ; Baxalta, now part of Shire; Bayer; Bioverativ; CSL; Novo Nordisk, Roche and Sobi with administrative support provided by the US National Hemophilia Foundation: Research Funding; US National Hemophilia Foundation: Other: non-financial support . Curtis: Bayer: Research Funding, Speakers Bureau; Bioverativ: Research Funding; Genentech: Honoraria, Research Funding; Gilead: Honoraria; Pfizer: Research Funding; Novo Nordisk: Honoraria, Research Funding; Baxter: Research Funding; CSL Behring: Research Funding. Noone: Baxalta, now part of Shire; Bayer; Bioverativ; CSL; Novo Nordisk, Roche and Sobi with administrative support provided by the US National Hemophilia Foundation: Research Funding. O'Mahony: US National Hemophilia Foundation: Other: non-financial support.
Author notes
Asterisk with author names denotes non-ASH members.